Which Cell Junction Is Unique To Animals
| Cell junction | |
|---|---|
| Details | |
| Identifiers | |
| Latin | junctiones cellulares |
| Thursday | H1.00.01.0.00012 |
| FMA | 67394 |
| Anatomical terminology [edit on Wikidata] | |
Jail cell junctions (or intercellular bridges [1]) are a class of cellular structures consisting of multiprotein complexes that provide contact or adhesion between neighboring cells or betwixt a jail cell and the extracellular matrix in animals. They also maintain the paracellular barrier of epithelia and control paracellular transport. Jail cell junctions are particularly abundant in epithelial tissues. Combined with prison cell adhesion molecules and extracellular matrix, cell junctions assistance hold animal cells together.
Jail cell junctions are likewise especially important in enabling communication between neighboring cells via specialized protein complexes called communicating (gap) junctions. Cell junctions are also of import in reducing stress placed upon cells.
In plants, similar advice channels are known as plasmodesmata, and in fungi they are chosen septal pores.[2]
Types [edit]

Some examples of jail cell junctions
In vertebrates, in that location are 3 major types of cell junction:
- Adherens junctions, desmosomes and hemidesmosomes (anchoring junctions)
- Gap junctions[3] (communicating junction)
- Tight junctions (occluding junctions)
Invertebrates have several other types of specific junctions, for example septate junctions or the C. elegans upmost junction. In multicellular plants, the structural functions of cell junctions are instead provided for by prison cell walls. The analogues of chatty prison cell junctions in plants are called plasmodesmata.
Anchoring junctions [edit]
Cells within tissues and organs must be anchored to one another and attached to components of the extracellular matrix. Cells take developed several types of junctional complexes to serve these functions, and in each case, anchoring proteins extend through the plasma membrane to link cytoskeletal proteins in ane cell to cytoskeletal proteins in neighboring cells every bit well every bit to proteins in the extracellular matrix.[4]
Three types of anchoring junctions are observed, and differ from 1 some other in the cytoskeletal poly peptide anchor every bit well as the transmembrane linker protein that extends through the membrane:
| Junction | Cytoskeletal anchor | Transmembrane linker | Ties cell to: |
|---|---|---|---|
| Desmosomes | Intermediate filaments | Cadherin | Other cells |
| Hemidesmosomes | Intermediate filaments | Integrins | EC matrix |
| Adherens junctions(Adhesion chugalug, Focal adhesion) | Actin filaments | Cadherin / Integrins | Other cells / EC matrix |
Anchoring-type junctions not only hold cells together but provide tissues with structural cohesion. These junctions are most abundant in tissues that are subject to constant mechanical stress such as peel and heart.[4]
Desmosomes [edit]

This image shows a desmosome junction betwixt cells of the epidermal layer of the peel.
Desmosomes, as well termed as maculae adherentes, can be visualized as rivets through the plasma membrane of adjacent cells. Intermediate filaments composed of keratin or desmin are attached to membrane-associated attachment proteins that form a dense plaque on the cytoplasmic face of the membrane. Cadherin molecules form the actual ballast by attaching to the cytoplasmic plaque, extending through the membrane and binding strongly to cadherins coming through the membrane of the adjacent cell.[five]
Hemidesmosomes [edit]
Hemidesmosomes form rivet-like links betwixt cytoskeleton and extracellular matrix components such as the basal laminae that underlie epithelia. Like desmosomes, they tie to intermediate filaments in the cytoplasm, merely in contrast to desmosomes, their transmembrane anchors are integrins rather than cadherins.[6]
Adherens junctions [edit]
Adherens junctions share the characteristic of anchoring cells through their cytoplasmic actin filaments. Similarly to desmosomes and hemidesmosomes, their transmembrane anchors are composed of cadherins in those that anchor to other cells and integrins (focal adhesion) in those that ballast to extracellular matrix. There is considerable morphologic multifariousness amid adherens junctions. Those that tie cells to one another are seen as isolated streaks or spots, or as bands that completely encircle the cell. The band-type of adherens junctions is associated with bundles of actin filaments that also encircle the cell but below the plasma membrane. Spot-similar adherens junctions chosen focal adhesions help cells adhere to extracellular matrix. The cytoskeletal actin filaments that tie into adherens junctions are contractile proteins and in addition to providing an anchoring part, adherens junctions are thought to participate in folding and angle of epithelial jail cell sheets. Thinking of the bands of actin filaments as beingness like to 'drawstrings' allows i to envision how contraction of the bands within a group of cells would distort the sheet into interesting patterns.[4]
Communicating (gap) junctions [edit]
Communicating junctions, or gap junctions permit for directly chemic communication between adjacent cellular cytoplasm through diffusion without contact with the extracellular fluid.[vii] This is possible due to six connexin proteins interacting to class a cylinder with a pore in the centre called a connexon.[8] The connexon complexes stretches across the cell membrane and when 2 adjacent cell connexons interact, they course a complete gap junction channel.[7] [viii] Connexon pores vary in size, polarity and therefore tin can be specific depending on the connexin proteins that constitute each private connexon.[7] [8] Whilst variation in gap junction channels do occur, their structure remains relatively standard, and this interaction ensures efficient advice without the escape of molecules or ions to the extracellular fluid.[8]
Gap junctions play vital roles in the human trunk,[ix] including their role in the compatible contractile of the centre muscle.[nine] They are likewise relevant in signal transfers in the brain, and their absence shows a decreased prison cell density in the brain.[10] Retinal and skin cells are also dependent on gap junctions in cell differentiation and proliferation.[ix] [ten]
Tight junctions [edit]
Found in vertebrate epithelia, tight junctions act every bit barriers that regulate the motion of water and solutes between epithelial layers. Tight junctions are classified as a paracellular barrier which is defined as not having directional discrimination; however, movement of the solute is largely dependent upon size and charge. There is prove to suggest that the structures in which solutes pass through are somewhat like pores.
Physiological pH plays a function in the selectivity of solutes passing through tight junctions with nearly tight junctions being slightly selective for cations. Tight junctions present in unlike types of epithelia are selective for solutes of differing size, charge, and polarity.
Proteins [edit]
In that location have been approximately 40 proteins identified to be involved in tight junctions. These proteins can be classified into four major categories; scaffolding proteins, signalling proteins, regulation proteins, and transmembrane proteins.
Roles [edit]
- Scaffolding proteins – organise the transmembrane proteins, couple transmembrane proteins to other cytoplasmic proteins as well equally to actin filaments.
- Signaling proteins – involved in junctions assembly, barrier regulation, and gene transcription.
- Regulation proteins – regulate membrane vesicle targeting.
- Transmembrane proteins – including junctional adhesion molecule, occludin, and claudin.
It is believed that claudin is the poly peptide molecule responsible for the selective permeability betwixt epithelial layers.
A three-dimensional image is still yet to be achieved and as such specific information most the function of tight junctions is still to be adamant.
Tricellular junctions [edit]
Tricellular junctions seal epithelia at the corners of iii cells. Due to the geometry of three-jail cell vertices, the sealing of the cells at these sites requires a specific junctional organization, different from those in bicellular junctions. In vertebrates, components tricellular junctions are tricellulin and lipolysis-stimulated lipoprotein receptors. In invertebrates, the components are gliotactin and anakonda.[11]
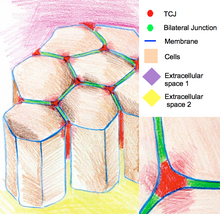
The cartoon of epithelium cells continued by tricellular junctions at the regions where three cells run into.
Tricellular junctions are also implicated in the regulation of cytoskeletal organization and cell divisions. In particular they ensure that cells separate co-ordinate to the Hertwig rule. In some Drosophila epithelia, during jail cell divisions tricellular junctions institute physical contact with spindle apparatus through astral microtubules. Tricellular junctions exert a pulling force on the spindle appliance and serve every bit a geometrical clues to decide orientation of cell divisions.[12]
Prison cell junction molecules [edit]
The molecules responsible for creating cell junctions include various cell adhesion molecules. There are 4 master types: selectins, cadherins, integrins, and the immunoglobulin superfamily.[13]
Selectins are prison cell adhesion molecules that play an important office in the initiation of inflammatory processes.[14] The functional capacity of selectin is limited to leukocyte collaborations with vascular endothelium. There are three types of selectins institute in humans; Fifty-selectin, P-selectin and East-selectin. L-selectin deals with lymphocytes, monocytes and neutrophils, P-selectin deals with platelets and endothelium and Due east-selectin deals only with endothelium. They have extracellular regions made up of an amino-last lectin domain, attached to a sugar ligand, growth factor-like domain, and short repeat units (numbered circles) that match the complementary bounden poly peptide domains.[15]
Cadherins are calcium-dependent adhesion molecules. Cadherins are extremely important in the procedure of morphogenesis – fetal development. Together with an alpha-beta catenin complex, the cadherin can bind to the microfilaments of the cytoskeleton of the cell. This allows for homophilic prison cell–cell adhesion.[16] The β-catenin–α-catenin linked complex at the adherens junctions allows for the formation of a dynamic link to the actin cytoskeleton.[17]
Integrins deed as adhesion receptors, transporting signals across the plasma membrane in multiple directions. These molecules are an invaluable part of cellular communication, as a unmarried ligand tin be used for many integrins. Unfortunately these molecules still have a long style to get in the ways of research.[xviii]
Immunoglobulin superfamily are a group of calcium contained proteins capable of homophilic and heterophilic adhesion. Homophilic adhesion involves the immunoglobulin-similar domains on the cell surface binding to the immunoglobulin-like domains on an opposing cell's surface while heterophilic adhesion refers to the binding of the immunoglobulin-like domains to integrins and carbohydrates instead.[19]
Cell adhesion is a vital component of the body. Loss of this adhesion furnishings cell structure, cellular performance and communication with other cells and the extracellular matrix and tin can lead to severe wellness issues and diseases.
References [edit]
- ^ Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul Chiliad.; Fausto, Nelson (2007). "Ch. thirteen: Box on morphology of squamous cell carcinoma". Robbins Basic Pathology (8th ed.). Philadelphia: Saunders. ISBN978-one-4160-2973-ane.
- ^ Bloemendal, S; Kück, U (January 2013). "Jail cell-to-cell advice in plants, animals, and fungi: a comparative review". Die Naturwissenschaften. 100 (one): 3–nineteen. Bibcode:2013NW....100....3B. doi:10.1007/s00114-012-0988-z. PMID 23128987. S2CID 11991859.
- ^ Andrew 50 Harris; Darren Locke (2009). Connexins, A Guide. New York: Springer. p. 574. ISBN978-ane-934115-46-half dozen.
- ^ a b c Yan HH, Mruk DD, Lee WM, Cheng CY (2008). Cantankerous-talk between tight and anchoring junctions-lesson from the testis . Advances in Experimental Medicine and Biology. Vol. 636. New York, NY : Springer-Verlag New York. pp. 234–54. doi:ten.1007/978-0-387-09597-4_13. ISBN978-0-387-79990-2. PMC4080640. PMID 19856171.
- ^ Lie PP, Cheng CY, Mruk DD (2011). The biological science of the desmosome-like junction a versatile anchoring junction and signal transducer in the seminiferous epithelium. International Review of Cell and Molecular Biology. Vol. 286. pp. 223–69. doi:10.1016/B978-0-12-385859-7.00005-7. ISBN9780123858597. PMC4381909. PMID 21199783.
- ^ Gipson IK, Spurr-Michaud SJ, Tisdale Equally (April 1988). "Hemidesmosomes and anchoring fibril collagen appear synchronously during development and wound healing". Developmental Biology. 126 (2): 253–62. doi:x.1016/0012-1606(88)90136-4. PMID 3350210.
- ^ a b c Evans WH, Martin PE (2002). "Gap junctions: structure and function (Review)". Molecular Membrane Biology. xix (2): 121–36. doi:x.1080/09687680210139839. PMID 12126230. S2CID 20806078.
- ^ a b c d Lampe PD, Lau AF (July 2004). "The effects of connexin phosphorylation on gap junctional advice". International Journal of Biochemistry & Cell Biology. 36 (7): 1171–86. doi:10.1016/S1357-2725(03)00264-4. PMC2878204. PMID 15109565.
- ^ a b c "Abstracts: Proceedings of the International Gap Junction Conference. August 5–nine, 2007. Elsinore, Denmark". Cell Communication & Adhesion. 14 (6): 275–346. 2007. doi:10.1080/15419060801891042. PMID 18392995.
- ^ a b Wei CJ, Xu X, Lo CW (2004). "Connexins and cell signaling in evolution and affliction". Almanac Review of Prison cell and Developmental Biology. 20: 811–38. doi:ten.1146/annurev.cellbio.19.111301.144309. PMID 15473861.
- ^ Byri S, Misra T, Syed ZA, Batz T, Shah J, Boril L, Glashauser J, Aegerter-Wilmsen T, Matzat T, Moussian B, Uv A, Luschnig S (2015). "The triple-repeat protein Anakonda controls epithelial tricellular junction germination in Drosophila". Developmental Jail cell. 33 (five): 535–48. doi:x.1016/j.devcel.2015.03.023. PMID 25982676.
- ^ Bosveld F, Markova O, Guirao B, Martin C, Wang Z, Pierre A, Balakireva G, Gaugue I, Ainslie A, Christophorou N, Lubensky DK, Minc Due north, Bellaïche Y (2016). "Epithelial tricellular junctions act as interphase prison cell shape sensors to orient mitosis". Nature. 530 (7591): 496–8. Bibcode:2016Natur.530..495B. doi:10.1038/nature16970. PMC5450930. PMID 26886796.
- ^ Lodish; et al. (2007). Molecular Cell Biological science (6th ed.). W. H. Freeman and Visitor. p. 803. ISBN978-1429203142.
- ^ Tedder TF, Steeber DA, Chen A, Engel P (July 1995). "The selectins: vascular adhesion molecules". FASEB Journal. ix (10): 866–73. doi:10.1096/fasebj.nine.10.7542213. PMID 7542213. S2CID 8315194.
- ^ Bevilacqua MP, Nelson RM (February 1993). "Selectins". Journal of Clinical Investigation. 91 (two): 379–87. doi:x.1172/JCI116210. PMC287934. PMID 7679406.
- ^ Rowlands TM, Symonds JM, Farookhi R, Blaschuk OW (January 2000). "Cadherins: crucial regulators of structure and role in reproductive tissues". Reviews of Reproduction. v (i): 53–61. doi:10.1530/revreprod/v.1.53. PMID 10711736.
- ^ Brembeck FH, Rosário M, Birchmeier W (Feb 2006). "Balancing cell adhesion and Wnt signaling, the fundamental role of β-catenin". Current Opinion in Genetics & Development. 16 (1): 51–9. doi:10.1016/j.gde.2005.12.007. PMID 16377174.
- ^ Hynes RO (September 2002). "Integrins: bidirectional, allosteric signaling machines". Cell. 110 (vi): 673–87. doi:ten.1016/S0092-8674(02)00971-six. PMID 12297042. S2CID 30326350.
- ^ Wai Wong C, Dye DE, Coombe DR (2012). "The function of immunoglobulin superfamily prison cell adhesion molecules in cancer metastasis". International Periodical of Cell Biology. 2012: 1–ix. doi:10.1155/2012/340296. PMC3261479. PMID 22272201.
External links [edit]
- Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2002). "Jail cell Junctions". Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN978-0-8153-3218-three.
- Intercellular+Junctions at the US National Library of Medicine Medical Subject Headings (MeSH)
- Cell-Matrix+Junctions at the United states National Library of Medicine Medical Subject Headings (MeSH)
Source: https://en.wikipedia.org/wiki/Cell_junction
Posted by: laneusety1965.blogspot.com

0 Response to "Which Cell Junction Is Unique To Animals"
Post a Comment