Where Is The Cytoskeleton Located In An Animal Cell
| Jail cell biology | |
|---|---|
| Animal cell diagram | |
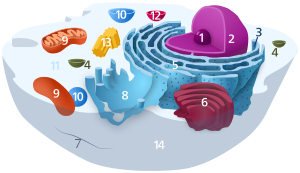 Components of a typical animal prison cell:
|

The cytoskeleton is a complex, dynamic network of interlinking poly peptide filaments present in the cytoplasm of all cells, excluding leaner and archaea.[1] Information technology extends from the jail cell nucleus to the cell membrane and is equanimous of similar proteins in the various organisms. In eukaryotes, it is equanimous of three primary components, microfilaments, intermediate filaments and microtubules, and these are all capable of rapid growth or disassembly dependent on the cell's requirements.[two]
A multitude of functions can be performed past the cytoskeleton. Its primary function is to give the prison cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells information technology stabilizes entire tissues.[three] [4] The cytoskeleton can too contract, thereby deforming the cell and the cell's surround and allowing cells to migrate.[5] Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material (endocytosis),[half-dozen] the segregation of chromosomes during cellular sectionalization,[iii] the cytokinesis stage of cell sectionalisation,[vii] as scaffolding to organize the contents of the cell in space[5] and in intracellular transport (for example, the movement of vesicles and organelles inside the cell)[3] and can be a template for the construction of a jail cell wall.[3] Furthermore, it can course specialized structures, such as flagella, cilia, lamellipodia and podosomes. The structure, function and dynamic behavior of the cytoskeleton can be very different, depending on organism and cell blazon.[3] [7] Even within one cell, the cytoskeleton tin change through association with other proteins and the previous history of the network.[v]
A large-scale example of an action performed by the cytoskeleton is muscle wrinkle. This is carried out by groups of highly specialized cells working together. A main component in the cytoskeleton that helps testify the true function of this muscle contraction is the microfilament. Microfilaments are composed of the most arable cellular protein known every bit actin.[8] During contraction of a muscle, within each muscle cell, myosin molecular motors collectively exert forces on parallel actin filaments. Muscle wrinkle starts from nerve impulses which then causes increased amounts of calcium to exist released from the sarcoplasmic reticulum. Increases in calcium in the cytosol allows muscle contraction to begin with the help of two proteins, tropomyosin and troponin.[8] Tropomyosin inhibits the interaction between actin and myosin, while troponin senses the increase in calcium and releases the inhibition.[9] This action contracts the muscle jail cell, and through the synchronous process in many muscle cells, the entire muscle.
History [edit]
In 1903, Nikolai Yard. Koltsov proposed that the shape of cells was determined by a network of tubules that he termed the cytoskeleton. The concept of a protein mosaic that dynamically coordinated cytoplasmic biochemistry was proposed by Rudolph Peters in 1929[10] while the term (cytosquelette, in French) was get-go introduced by French embryologist Paul Wintrebert in 1931.[11]
When the cytoskeleton was outset introduced, information technology was thought to be an uninteresting gel-like substance that helped organelles stay in place.[12] Much research took place to try to understand the purpose of the cytoskeleton and its components. With the help of Stuart Hameroff and Roger Penrose, it was discovered that microtubules vibrate inside neurons in the brain, suggesting that brain waves come from deeper microtubule vibrations.[13] This discovery demonstrated that the cytoskeleton is not just a gel-like substance and that information technology really has a purpose.[ disputed ]
Initially, it was thought that the cytoskeleton was sectional to eukaryotes but in 1992 it was discovered to be present in prokaryotes likewise. This discovery came later the realization that bacteria possess proteins that are homologous to tubulin and actin; the master components of the eukaryotic cytoskeleton.[14]
Eukaryotic cytoskeleton [edit]
Eukaryotic cells incorporate three main kinds of cytoskeletal filaments: microfilaments, microtubules, and intermediate filaments. In neurons the intermediate filaments are known as neurofilaments.[15] Each type is formed past the polymerization of a distinct type of poly peptide subunit and has its own characteristic shape and intracellular distribution. Microfilaments are polymers of the protein actin and are 7 nm in diameter. Microtubules are composed of tubulin and are 25 nm in diameter. Intermediate filaments are composed of various proteins, depending on the type of cell in which they are found; they are normally eight-12 nm in diameter.[one] The cytoskeleton provides the cell with structure and shape, and past excluding macromolecules from some of the cytosol, it adds to the level of macromolecular crowding in this compartment.[16] Cytoskeletal elements interact extensively and intimately with cellular membranes.[17]
Research into neurodegenerative disorders such as Parkinson's disease, Alzheimer's disease, Huntington's disease, and amyotrophic lateral sclerosis (ALS) indicate that the cytoskeleton is affected in these diseases.[18] Parkinson's affliction is marked past the deposition of neurons, resulting in tremors, rigidity, and other non-motor symptoms. Research has shown that microtubule associates and stability in the cytoskeleton is compromised causing the neurons to dethrone over fourth dimension.[19] In Alzheimer's illness, tau proteins which stabilize microtubules malfunction in the progression of the illness causing pathology of the cytoskeleton.[20] Excess glutamine in the Huntington protein involved with linking vesicles onto the cytoskeleton is also proposed to be a factor in the development of Huntington's Disease.[21] Amyotrophic Lateral Sclerosis results in a loss of movement caused by the degradation of motor neurons, and too involves defects of the cytoskeleton.[22]
Accompaniment proteins including motor proteins regulate and link the filaments to other jail cell compounds and each other and are essential for controlled assembly of cytoskeletal filaments in particular locations.[23]
A number of small-scale-molecule cytoskeletal drugs accept been discovered that interact with actin and microtubules. These compounds have proven useful in studying the cytoskeleton, and several have clinical applications.
Microfilaments [edit]
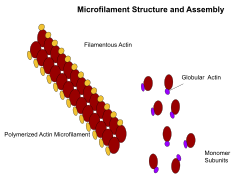

Microfilaments, also known as actin filaments, are equanimous of linear polymers of Chiliad-actin proteins, and generate force when the growing (plus) finish of the filament pushes confronting a barrier, such every bit the cell membrane. They also human action as tracks for the movement of myosin molecules that affix to the microfilament and "walk" along them. In general, the major component or protein of microfilaments are actin. The K-actin monomer combines to grade a polymer which continues to form the microfilament (actin filament). These subunits then get together into two chains that intertwine into what are called F-actin chains.[24] Myosin motoring forth F-actin filaments generates contractile forces in so-called actomyosin fibers, both in muscle as well as most non-muscle cell types.[25] Actin structures are controlled by the Rho family of small GTP-binding proteins such equally Rho itself for contractile acto-myosin filaments ("stress fibers"), Rac for lamellipodia and Cdc42 for filopodia.
Functions include:
- Muscle wrinkle
- Prison cell movement
- Intracellular transport/trafficking
- Maintenance of eukaryotic cell shape
- Cytokinesis
- Cytoplasmic streaming[24]
Intermediate filaments [edit]

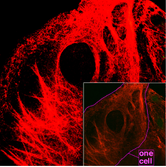
Microscopy of keratin filaments within cells
Intermediate filaments are a part of the cytoskeleton of many eukaryotic cells. These filaments, averaging 10 nanometers in diameter, are more stable (strongly bound) than microfilaments, and heterogeneous constituents of the cytoskeleton. Similar actin filaments, they office in the maintenance of cell-shape by bearing tension (microtubules, by dissimilarity, resist compression merely tin can too bear tension during mitosis and during the positioning of the centrosome). Intermediate filaments organize the internal tridimensional construction of the cell, anchoring organelles and serving as structural components of the nuclear lamina. They also participate in some cell-cell and prison cell-matrix junctions. Nuclear lamina exist in all animals and all tissues. Some animals like the fruit fly exercise not have any cytoplasmic intermediate filaments. In those animals that express cytoplasmic intermediate filaments, these are tissue specific.[4] Keratin intermediate filaments in epithelial cells provide protection for dissimilar mechanical stresses the pare may suffer. They also provide protection for organs against metabolic, oxidative, and chemical stresses. Strengthening of epithelial cells with these intermediate filaments may preclude onset of apoptosis, or cell death, by reducing the probability of stress.[26]
Intermediate filaments are most commonly known as the support arrangement or "scaffolding" for the cell and nucleus while also playing a office in some cell functions. In combination with proteins and desmosomes, the intermediate filaments form jail cell-jail cell connections and anchor the cell-matrix junctions that are used in messaging betwixt cells too equally vital functions of the cell. These connections permit the cell to communicate through the desmosome of multiple cells to suit structures of the tissue based on signals from the cells environment. Mutations in the IF proteins have been shown to cause serious medical problems such as premature crumbling, desmin mutations compromising organs, Alexander Disease, and muscular dystrophy.[27]
Different intermediate filaments are:
- made of vimentins. Vimentin intermediate filaments are in general present in mesenchymal cells.
- made of keratin. Keratin is nowadays in general in epithelial cells.
- neurofilaments of neural cells.
- made of lamin, giving structural support to the nuclear envelope.
- fabricated of desmin, play an important role in structural and mechanical support of muscle cells.[28]
Microtubules [edit]

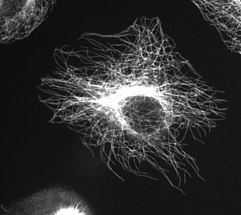
Microtubules in a gel-fixated cell
Microtubules are hollow cylinders about 23 nm in diameter (lumen diameter of approximately xv nm), most commonly comprising 13 protofilaments that, in turn, are polymers of alpha and beta tubulin. They have a very dynamic behavior, binding GTP for polymerization. They are commonly organized by the centrosome.
In nine triplet sets (star-shaped), they form the centrioles, and in nine doublets oriented about two additional microtubules (bicycle-shaped), they form cilia and flagella. The latter germination is unremarkably referred to as a "9+2" organisation, wherein each doublet is connected to another by the protein dynein. As both flagella and cilia are structural components of the cell, and are maintained past microtubules, they tin can be considered part of the cytoskeleton. At that place are two types of cilia: motile and non-motile cilia. Cilia are short and more numerous than flagella. The motile cilia have a rhythmic waving or beating motion compared to the non-motile cilia which receive sensory information for the cell; processing signals from the other cells or the fluids surrounding information technology. Additionally, the microtubules control the beating (movement) of the cilia and flagella.[29] Too, the dynein arms fastened to the microtubules office every bit the molecular motors. The motion of the cilia and flagella is created past the microtubules sliding past one some other, which requires ATP.[29] They play key roles in:
- intracellular transport (associated with dyneins and kinesins, they transport organelles like mitochondria or vesicles).
- the axoneme of cilia and flagella.

Cross section diagram through the cilium, showing the "9 + ii" organization of microtubules
- the mitotic spindle.
- synthesis of the cell wall in plants.
In add-on to the roles described higher up, Stuart Hameroff and Roger Penrose take proposed that microtubules role in consciousness.[30]
Comparison [edit]
| Cytoskeleton type[31] | Bore (nm)[32] | Structure | Subunit examples[31] |
|---|---|---|---|
| Microfilaments | six | Double helix | Actin |
| Intermediate filaments | 10 | 2 anti-parallel helices/dimers, forming tetramers |
|
| Microtubules | 23 | Protofilaments, in turn consisting of tubulin subunits in complex with stathmin[33] | α- and β-Tubulin |
Septins [edit]
Septins are a group of the highly conserved GTP bounden proteins plant in eukaryotes. Dissimilar septins form protein complexes with each other. These tin assemble to filaments and rings. Therefore, septins tin can exist considered part of the cytoskeleton.[34] The function of septins in cells include serving as a localized attachment site for other proteins, and preventing the diffusion of certain molecules from one jail cell compartment to another.[34] In yeast cells, they build scaffolding to provide structural support during cell division and compartmentalize parts of the cell. Recent enquiry in human cells suggests that septins build cages around bacterial pathogens, immobilizing the harmful microbes and preventing them from invading other cells.[35]
Spectrin [edit]
Spectrin is a cytoskeletal poly peptide that lines the intracellular side of the plasma membrane in eukaryotic cells. Spectrin forms pentagonal or hexagonal arrangements, forming a scaffolding and playing an of import part in maintenance of plasma membrane integrity and cytoskeletal construction.[36]
Yeast cytoskeleton [edit]
In budding yeast (an important model organism), actin forms cortical patches, actin cables, and a cytokinetic band and the cap. Cortical patches are detached actin bodies on the membrane and are vital for endocytosis, especially the recycling of glucan synthase which is important for jail cell wall synthesis. Actin cables are bundles of actin filaments and are involved in the transport of vesicles towards the cap (which contains a number of different proteins to polarize cell growth) and in the positioning of mitochondria. The cytokinetic band forms and constricts around the site of cell division.[37]
Prokaryotic cytoskeleton [edit]
Prior to the work of Jones et al., 2001, the jail cell wall was believed to exist the deciding factor for many bacterial cell shapes, including rods and spirals. When studied, many misshapen leaner were constitute to have mutations linked to evolution of a cell envelope.[38] The cytoskeleton was once idea to be a characteristic only of eukaryotic cells, but homologues to all the major proteins of the eukaryotic cytoskeleton accept been found in prokaryotes.[39] Harold Erickson notes that earlier 1992, only eukaryotes were believed to have cytoskeleton components. However, enquiry in the early '90s suggested that leaner and archaea had homologues of actin and tubulin, and that these were the basis of eukaryotic microtubules and microfilaments.[40] Although the evolutionary relationships are and so distant that they are not obvious from protein sequence comparisons lone, the similarity of their three-dimensional structures and similar functions in maintaining cell shape and polarity provides strong evidence that the eukaryotic and prokaryotic cytoskeletons are truly homologous.[41] Three laboratories independently discovered that FtsZ, a poly peptide already known as a central player in bacterial cytokinesis, had the "tubulin signature sequence" present in all α-, β-, and γ-tubulins.[40] However, some structures in the bacterial cytoskeleton may non have been identified as of nevertheless.[25] [42]
FtsZ [edit]
FtsZ was the showtime protein of the prokaryotic cytoskeleton to be identified. Like tubulin, FtsZ forms filaments in the presence of guanosine triphosphate (GTP), simply these filaments do not grouping into tubules. During cell division, FtsZ is the first protein to move to the partitioning site, and is essential for recruiting other proteins that synthesize the new cell wall betwixt the dividing cells.
MreB and ParM [edit]
Prokaryotic actin-similar proteins, such as MreB, are involved in the maintenance of cell shape. All non-spherical leaner have genes encoding actin-like proteins, and these proteins form a helical network beneath the cell membrane that guides the proteins involved in cell wall biosynthesis.[43]
Some plasmids encode a separate system that involves an actin-like protein ParM. Filaments of ParM exhibit dynamic instability, and may partition plasmid Dna into the dividing daughter cells by a mechanism analogous to that used past microtubules during eukaryotic mitosis.[25] [44]
Crescentin [edit]
The bacterium Caulobacter crescentus contains a 3rd protein, crescentin, that is related to the intermediate filaments of eukaryotic cells. Crescentin is too involved in maintaining cell shape, such as helical and vibrioid forms of bacteria, but the machinery by which information technology does this is currently unclear.[45] Additionally, curvature could be described by the displacement of crescentic filaments, later on the disruption of peptidoglycan synthesis.[46]
The cytoskeleton and prison cell mechanics [edit]
The cytoskeleton is a highly anisotropic and dynamic network, constantly remodeling itself in response to the irresolute cellular microenvironment. The network influences cell mechanics and dynamics by differentially polymerizing and depolymerizing its elective filaments (primarily actin and myosin, just microtubules and intermediate filaments also play a role).[47] This generates forces, which play an important role in informing the cell of its microenvironment. Specifically, forces such equally tension, stiffness, and shear forces have all been shown to influence cell fate, differentiation, migration, and motility.[48] Through a process called "mechanotransduction," the cell remodels its cytoskeleton to sense and respond to these forces.
[[Mechanotransduction]] relies heavily on [[focal adhesions]], which essentially connect the intracellular cytoskeleton with the [extracellular matrix] (ECM). Through [focal adhesions], the cell is able to integrate extracellular forces into intracellular ones every bit the proteins present at focal adhesions undergo conformational changes to initiate signaling cascades. Proteins such equally focal adhesion kinase (FAK) and Src have been shown to transduce force signals in response to cellular activities such every bit proliferation and differentiation, and are hypothesized to exist key sensors in the mechanotransduction pathway.[49] As a result of mechanotransduction, the cytoskeleton changes its limerick and/or orientation to suit the strength stimulus and ensure the cell responds appropriately.
The cytoskeleton changes the mechanics of the cell in response to detected forces. For case, increasing tension within the plasma membrane makes information technology more than likely that ion channels will open up, which increases ion conductance and makes cellular change ion influx or efflux much more than likely.[50] Moreover, the mechanical properties of cells determine how far and where, directionally, a strength will propagate throughout the cell and how it will change cell dynamics.[51] A membrane protein that is not closely coupled to the cytoskeleton, for instance, will not produce a significant effect on the cortical actin network if it is subjected to a specifically directed strength. However, membrane proteins that are more closely associated with the cytoskeleton will induce a more than significant response.[52] In this manner, the anisotropy of the cytoskeleton serves to more keenly direct cell responses to intra or extracellular signals.
Long range society [edit]
The specific pathways and mechanisms by which the cytoskeleton senses and responds to forces are withal under investigation. Even so, the [[long range order]] generated by the cytoskeleton is known to contribute to mechanotransduction.[53] Cells, which are around 10-50 microns in diameter, are several yard times larger than the molecules found within the cytoplasm that are essential to coordinate cellular activities. Because cells are so large in comparison to essential biomolecules, information technology is difficult, in the absence of an organizing network, for different parts of the cytoplasm to communicate.[54] Moreover, biomolecules must polymerize to lengths comparable to the length of the cell, but resulting polymers can be highly disorganized and unable to finer transmit signals from one part of the cytoplasm to another. Thus, information technology is necessary to have the cytoskeleton to organize the polymers and ensure they tin can effectively communicate beyond the entirety of the jail cell.
Common features and differences between prokaryotes and eukaryotes [edit]
By definition, the cytoskeleton is composed of proteins that can form longitudinal arrays (fibres) in all organisms. These filament forming proteins have been classified into 4 classes. Tubulin-like, actin-like, Walker A cytoskeletal ATPases (WACA-proteins), and intermediate filaments.[7] [25]
Tubulin-like proteins are tubulin in eukaryotes and FtsZ, TubZ, RepX in prokaryotes. Actin-similar proteins are actin in eukaryotes and MreB, FtsA in prokaryotes. An example of a WACA-proteins, which are more often than not found in prokaryotes, is Heed. Examples for intermediate filaments, which have nigh exclusively been constitute in animals (i.eastward. eukaryotes) are the lamins, keratins, vimentin, neurofilaments, and desmin.[7]
Although tubulin-like proteins share some amino acid sequence similarity, their equivalence in protein-fold and the similarity in the GTP bounden site is more than striking. The same holds truthful for the actin-similar proteins and their structure and ATP bounden domain.[7] [25]
Cytoskeletal proteins are usually correlated with cell shape, Dna segregation and cell division in prokaryotes and eukaryotes. Which proteins fulfill which task is very different. For instance, Dna segregation in all eukaryotes happens through utilize of tubulin, only in prokaryotes either WACA proteins, actin-like or tubulin-similar proteins tin can be used. Cell division is mediated in eukaryotes past actin, just in prokaryotes usually by tubulin-like (often FtsZ-ring) proteins and sometimes (Thermoproteota) ESCRT-III, which in eukaryotes nevertheless has a function in the last step of division.[7]
Cytoplasmic streaming [edit]
Motion of organelles in Tradescantia stamen hair cells
Cytoplasmic streaming, also known as cyclosis, is the agile movement of a cell's contents along the components of the cytoskeleton. While mainly seen in plants, all cell types employ this process for transportation of waste, nutrients, and organelles to other parts of the cell.[55] Plant and algae cells are by and large larger than many other cells; and so cytoplasmic streaming is of import in these types of cells. This is because the cell's extra volume requires cytoplasmic streaming in order to motion organelles throughout the entire jail cell.[56] Organelles move along microfilaments in the cytoskeleton driven by myosin motors binding and pushing along actin filament bundles.[55]
Meet also [edit]
- Nuclear matrix – Fibrillar network lying on nuclear membrane
- Prison cell cortex – Layer on the inner face up of a prison cell membrane
References [edit]
- ^ a b Hardin J, Bertoni G, Kleinsmith LJ (2015). Becker's World of the Prison cell (8th ed.). New York: Pearson. pp. 422–446. ISBN978013399939-half-dozen.
- ^ McKinley, Michael; Dean O'Loughlin, Valerie; Pennefather-O'Brien, Elizabeth; Harris, Ronald (2015). Human Beefcake (4th ed.). New York: McGraw Hill Instruction. p. 29. ISBN978-0-07-352573-0.
- ^ a b c d due east Alberts B, et al. (2008). Molecular Biology of the Cell (5th ed.). New York: Garland Science. ISBN978-0-8153-4105-5.
- ^ a b Herrmann H, Bär H, Kreplak L, Strelkov SV, Aebi U (July 2007). "Intermediate filaments: from cell architecture to nanomechanics". Nature Reviews. Molecular Cell Biological science. 8 (7): 562–73. doi:10.1038/nrm2197. PMID 17551517. S2CID 27115011.
- ^ a b c Fletcher DA, Mullins RD (January 2010). "Cell mechanics and the cytoskeleton". Nature. 463 (7280): 485–92. Bibcode:2010Natur.463..485F. doi:ten.1038/nature08908. PMC2851742. PMID 20110992.
- ^ Geli MI, Riezman H (Apr 1998). "Endocytic internalization in yeast and animal cells: similar and dissimilar". Periodical of Cell Science. 111 ( Pt eight) (8): 1031–7. doi:10.1242/jcs.111.8.1031. PMID 9512499.
- ^ a b c d east f Wickstead B, Gull K (Baronial 2011). "The development of the cytoskeleton". The Periodical of Prison cell Biology. 194 (4): 513–25. doi:x.1083/jcb.201102065. PMC3160578. PMID 21859859.
- ^ a b Cooper, Geoffrey One thousand. (2000). "Actin, Myosin, and Cell Motility". The Jail cell: A Molecular Approach. 2nd Edition. Archived from the original on 2018-04-28.
- ^ Berg JM, Tymoczko JL, Stryer L (2002). "Myosins Movement Along Actin Filaments". Biochemistry. 5th Edition. Archived from the original on 2018-05-02.
- ^ Peters RA. "The Harben Lectures, 1929. Reprinted in: Peters, R. A. (1963) Biochemical lesions and lethal synthesis, p. 216. Pergamon Press, Oxford".
- ^ Frixione East (June 2000). "Recurring views on the structure and function of the cytoskeleton: a 300-year ballsy". Prison cell Motility and the Cytoskeleton. 46 (ii): 73–94. doi:10.1002/1097-0169(200006)46:two<73::Assist-CM1>three.0.CO;2-0. PMID 10891854. S2CID 16728876.
- ^ Hardin J (2015-12-03). Becker'due south World of the Prison cell (9th ed.). Pearson. p. 351. ISBN978-0-321-93492-5.
- ^ Elsevier. "Discovery of Breakthrough Vibrations in "Microtubules" Inside Brain Neurons Corroborates Controversial 20-Year-Onetime Theory of Consciousness". www.elsevier.com. Archived from the original on 2016-11-07. Retrieved 2017-11-20 .
- ^ Wickstead B, Dupe K (August 2011). "The evolution of the cytoskeleton". The Periodical of Prison cell Biology. 194 (iv): 513–25. doi:10.1083/jcb.201102065. PMC3160578. PMID 21859859.
- ^ Taran, Every bit; Shuvalova, LD; Lagarkova, MA; Alieva, IB (22 June 2020). "Huntington'southward Illness-An Outlook on the Interplay of the HTT Protein, Microtubules and Actin Cytoskeletal Components". Cells. 9 (6): 1514. doi:ten.3390/cells9061514. PMC7348758. PMID 32580314.
- ^ Minton AP (October 1992). "Solitude as a determinant of macromolecular structure and reactivity". Biophysical Journal. 63 (4): 1090–100. Bibcode:1992BpJ....63.1090M. doi:x.1016/S0006-3495(92)81663-6. PMC1262248. PMID 1420928.
- ^ Doherty GJ, McMahon HT (2008). "Mediation, modulation, and consequences of membrane-cytoskeleton interactions". Annual Review of Biophysics. 37: 65–95. doi:ten.1146/annurev.biophys.37.032807.125912. PMID 18573073. S2CID 17352662.
- ^ Pelucchi, Silvia; Stringhi, Ramona; Marcello, Elena (2020). "Dendritic Spines in Alzheimer's Disease: How the Actin Cytoskeleton Contributes to Synaptic Failure". International Journal of Molecular Sciences. 21 (iii): 908. doi:ten.3390/ijms21030908. ISSN 1422-0067. PMC7036943. PMID 32019166.
- ^ Pellegrini Fifty, Wetzel A, Grannó South, Heaton Yard, Harvey K (February 2017). "Back to the tubule: microtubule dynamics in Parkinson's affliction". Cellular and Molecular Life Sciences. 74 (3): 409–434. doi:10.1007/s00018-016-2351-half dozen. PMC5241350. PMID 27600680.
- ^ Bamburg JR, Bloom GS (August 2009). "Cytoskeletal pathologies of Alzheimer's Affliction". Cell Motility and the Cytoskeleton. 66 (8): 635–49. doi:10.1002/cm.20388. PMC2754410. PMID 19479823.
- ^ Caviston JP, Holzbaur EL (April 2009). "Huntingtin protein is an essential integrator of intracellular vesicular trafficking". Trends in Cell Biology. xix (4): 147–55. doi:x.1016/j.tcb.2009.01.005. PMC2930405. PMID 19269181.
- ^ Julien JP, Millecamps S, Kriz J (2005). "Cytoskeletal Defects in Amyotrophic Lateral Sclerosis (motor neuron affliction)". Novartis Foundation Symposium. 264: 183–92, word 192–half dozen, 227–xxx. PMID 15773754.
- ^ Alberts, Bruce (2015). Molecular Biology of the Cell. Garland Science. p. 889. ISBN978-0-8153-4464-3.
- ^ a b Cooper, Geoffrey 1000. (2000). "Structure and Organization of Actin Filaments". The Cell: A Molecular Approach. 2nd Edition. Archived from the original on 2018-05-02.
- ^ a b c d due east Gunning Pw, Ghoshdastider U, Whitaker S, Popp D, Robinson RC (June 2015). "The evolution of compositionally and functionally distinct actin filaments". Periodical of Cell Scientific discipline. 128 (11): 2009–19. doi:ten.1242/jcs.165563. PMID 25788699.
- ^ Pan X, Hobbs RP, Coulombe PA (February 2013). "The expanding significance of keratin intermediate filaments in normal and diseased epithelia". Electric current Stance in Cell Biology. 25 (ane): 47–56. doi:10.1016/j.ceb.2012.ten.018. PMC3578078. PMID 23270662.
- ^ Herrmann H, Bär H, Kreplak L, Strelkov SV, Aebi U (July 2007). "Intermediate filaments: from cell architecture to nanomechanics". Nature Reviews. Molecular Cell Biology. eight (vii): 562–73. doi:ten.1038/nrm2197. PMID 17551517. S2CID 27115011.
- ^ Paulin D, Li Z (November 2004). "Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle". Experimental Cell Research. 301 (1): 1–seven. doi:10.1016/j.yexcr.2004.08.004. PMID 15501438.
- ^ a b Lodish, Harvey; Berk, Arnold; Zipursky, S. Lawrence; Matsudaira, Paul; Baltimore, David; Darnell, James (two May 2018). "Cilia and Flagella: Construction and Movement". Archived from the original on two May 2018. Retrieved ii May 2018 – via world wide web.ncbi.nlm.nih.gov.
- ^ Hameroff, S. and Penrose, R. Physics of Life Reviews 2014, 11, 39-78
- ^ a b Unless else specified in boxes, then ref is:Boron WF (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 1300. ISBN978-1-4160-2328-ix. Page 25
- ^ Fuchs Eastward, Cleveland DW (January 1998). "A structural scaffolding of intermediate filaments in health and affliction". Science. 279 (5350): 514–9. Bibcode:1998Sci...279..514F. doi:x.1126/science.279.5350.514. PMID 9438837.
- ^ Steinmetz MO (May 2007). "Structure and thermodynamics of the tubulin-stathmin interaction". Journal of Structural Biology. 158 (2): 137–47. doi:10.1016/j.jsb.2006.07.018. PMID 17029844.
- ^ a b Mostowy S, Cossart P (February 2012). "Septins: the fourth component of the cytoskeleton". Nature Reviews. Molecular Cell Biology. xiii (iii): 183–94. doi:10.1038/nrm3284. PMID 22314400. S2CID 2418522.
- ^ Mascarelli A (December 2011). "Septin proteins take bacterial prisoners: A cellular defence against microbial pathogens holds therapeutic potential". Nature. doi:10.1038/nature.2011.9540. S2CID 85080734.
- ^ Huh GY, Glantz SB, Je S, Morrow JS, Kim JH (December 2001). "Calpain proteolysis of alpha 2-spectrin in the normal developed human brain". Neuroscience Messages. 316 (one): 41–4. doi:10.1016/S0304-3940(01)02371-0. PMID 11720774. S2CID 53270680.
- ^ Pruyne D, Bretscher A (February 2000). "Polarization of cell growth in yeast". Journal of Cell Science. 113 ( Pt four) (4): 571–85. doi:ten.1242/jcs.113.4.571. PMID 10652251.
- ^ Jones, Laura J. F.; Carballido-López, Rut; Errington, Jeffery (2001-03-23). "Control of Cell Shape in Bacteria: Helical, Actin-like Filaments in Bacillus subtilis". Cell. 104 (half dozen): 913–922. doi:10.1016/S0092-8674(01)00287-2. PMID 11290328. S2CID 14207533.
- ^ Shih YL, Rothfield L (September 2006). "The bacterial cytoskeleton". Microbiology and Molecular Biology Reviews. lxx (3): 729–54. doi:10.1128/MMBR.00017-06. PMC1594594. PMID 16959967.
- ^ a b Erickson HP (February 2017). "The discovery of the prokaryotic cytoskeleton: 25th anniversary". Molecular Biology of the Jail cell. 28 (3): 357–358. doi:10.1091/mbc.E16-03-0183. PMC5341718. PMID 28137947.
- ^ Michie KA, Löwe J (2006). "Dynamic filaments of the bacterial cytoskeleton" (PDF). Annual Review of Biochemistry. 75: 467–92. doi:ten.1146/annurev.biochem.75.103004.142452. PMID 16756499.
- ^ Briegel A, Dias DP, Li Z, Jensen RB, Frangakis Every bit, Jensen GJ (October 2006). "Multiple large filament bundles observed in Caulobacter crescentus past electron cryotomography". Molecular Microbiology. 62 (i): 5–14. doi:10.1111/j.1365-2958.2006.05355.x. PMID 16987173.
- ^ Popp D, Narita A, Maeda K, Fujisawa T, Ghoshdastider U, Iwasa M, Maéda Y, Robinson RC (May 2010). "Filament structure, organisation, and dynamics in MreB sheets". The Journal of Biological Chemical science. 285 (21): 15858–65. doi:10.1074/jbc.M109.095901. PMC2871453. PMID 20223832.
- ^ Popp D, Narita A, Lee LJ, Ghoshdastider U, Xue B, Srinivasan R, Balasubramanian MK, Tanaka T, Robinson RC (June 2012). "Novel actin-similar filament structure from Clostridium tetani". The Journal of Biological Chemistry. 287 (25): 21121–nine. doi:10.1074/jbc.M112.341016. PMC3375535. PMID 22514279.
- ^ Ausmees Due north, Kuhn JR, Jacobs-Wagner C (December 2003). "The bacterial cytoskeleton: an intermediate filament-like role in cell shape". Cell. 115 (6): 705–13. doi:x.1016/S0092-8674(03)00935-8. PMID 14675535. S2CID 14459851.
- ^ Esue, Osigwe (January 2010). "Dynamics of the Bacterial Intermediate Filament Crescentin In Vitro and In Vivo". PLOS I. 5 (one): e8855. Bibcode:2010PLoSO...5.8855E. doi:ten.1371/periodical.pone.0008855. PMC2816638. PMID 20140233. Retrieved 12 September 2017.
- ^ Chen, Christopher S. (2008-10-15). "Mechanotransduction – a field pulling together?". Periodical of Prison cell Science. 121 (20): 3285–3292. doi:10.1242/jcs.023507. ISSN 1477-9137. PMID 18843115. S2CID 1287523.
- ^ Chen, Christopher Southward. (2008-x-xv). "Mechanotransduction – a field pulling together?". Periodical of Cell Scientific discipline. 121 (20): 3285–3292. doi:10.1242/jcs.023507. ISSN 1477-9137. PMID 18843115. S2CID 1287523.
- ^ Orr, A. Wayne; Helmke, Brian P.; Blackman, Brett R.; Schwartz, Martin A. (January 2006). "Mechanisms of Mechanotransduction". Developmental Jail cell. 10 (1): xi–xx. doi:10.1016/j.devcel.2005.12.006. PMID 16399074.
- ^ Orr, A. Wayne; Helmke, Brian P.; Blackman, Brett R.; Schwartz, Martin A. (January 2006). "Mechanisms of Mechanotransduction". Developmental Cell. 10 (ane): 11–twenty. doi:ten.1016/j.devcel.2005.12.006. PMID 16399074.
- ^ Janmey, Paul A.; McCulloch, Christopher A. (2007-08-15). "Cell Mechanics: Integrating Cell Responses to Mechanical Stimuli". Annual Review of Biomedical Engineering. nine (1): 1–34. doi:10.1146/annurev.bioeng.ix.060906.151927. ISSN 1523-9829. PMID 17461730.
- ^ Orr, A. Wayne; Helmke, Brian P.; Blackman, Brett R.; Schwartz, Martin A. (January 2006). "Mechanisms of Mechanotransduction". Developmental Jail cell. 10 (ane): 11–20. doi:10.1016/j.devcel.2005.12.006. PMID 16399074.
- ^ Fletcher, Daniel A.; Mullins, R. Dyche (January 2010). "Cell mechanics and the cytoskeleton". Nature. 463 (7280): 485–492. Bibcode:2010Natur.463..485F. doi:x.1038/nature08908. ISSN 0028-0836. PMC2851742. PMID 20110992.
- ^ Mullins, R. D. (2010-01-01). "Cytoskeletal Mechanisms for Breaking Cellular Symmetry". Common cold Spring Harbor Perspectives in Biology. 2 (i): a003392. doi:x.1101/cshperspect.a003392. ISSN 1943-0264. PMC2827899. PMID 20182610.
- ^ a b Woodhouse FG, Goldstein RE (August 2013). "Cytoplasmic streaming in plant cells emerges naturally by microfilament cocky-organisation". Proceedings of the National Academy of Sciences of the United States of America. 110 (35): 14132–vii. arXiv:1308.6422. Bibcode:2013PNAS..11014132W. doi:10.1073/pnas.1302736110. PMC3761564. PMID 23940314.
- ^ Goldstein RE, van de Meent JW (August 2015). "A concrete perspective on cytoplasmic streaming". Interface Focus. 5 (4): 20150030. doi:10.1098/rsfs.2015.0030. PMC4590424. PMID 26464789.
External links [edit]
- Cytoskeleton Monthly News and Blog
- MBInfo - Cytoskeleton Dynamics
- Cytoskeleton, Cell Motility and Motors - The Virtual Library of Biochemistry, Molecular Biology and Cell Biological science
- Cytoskeleton database, clinical trials, contempo literature, lab registry ...
- Animation of leukocyte adhesion (Animation with some images of actin and microtubule assembly and dynamics.)
- http://cellix.imba.oeaw.ac.at/ Cytoskeleton and cell motility including videos
- Open up access review commodity on the emergent complexity of the cytoskeleton (appeared in Advances in Physics, 2013)
Source: https://en.wikipedia.org/wiki/Cytoskeleton
Posted by: laneusety1965.blogspot.com

0 Response to "Where Is The Cytoskeleton Located In An Animal Cell"
Post a Comment